
Our cleanroom range
Compare our cleanrooms to find the most appropriate solution for you.
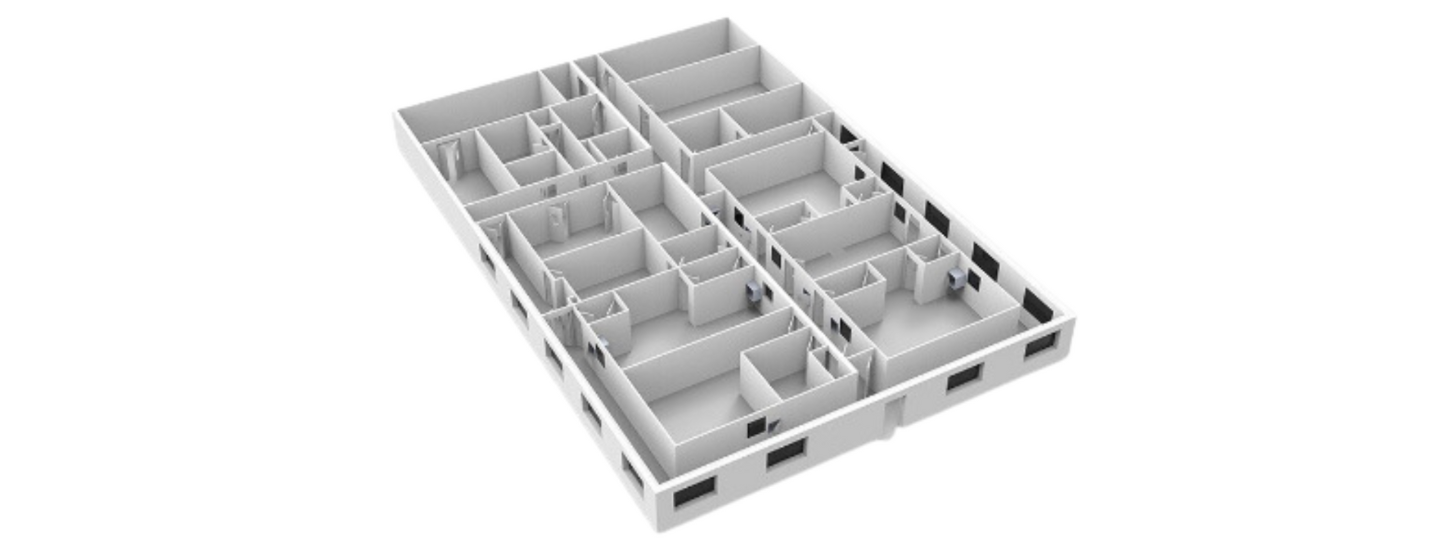
Custom-built GMP cleanrooms
- Diminishes the risks inherent to (bio)pharmaceutical production.
- Protects against viable and non-viable particulates, as well as cross-contamination.
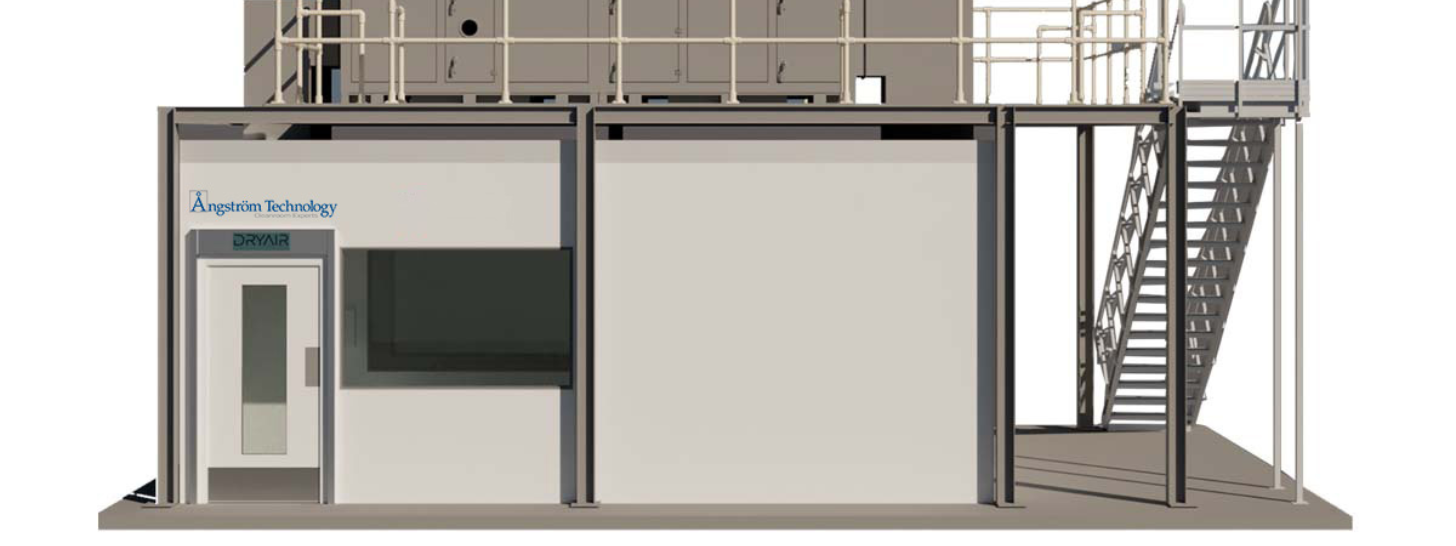
Custom-built ISO cleanrooms
- A repeatable environment for production of a reliable product.
- High-performance cleanrooms designed to interface with your existing facility.
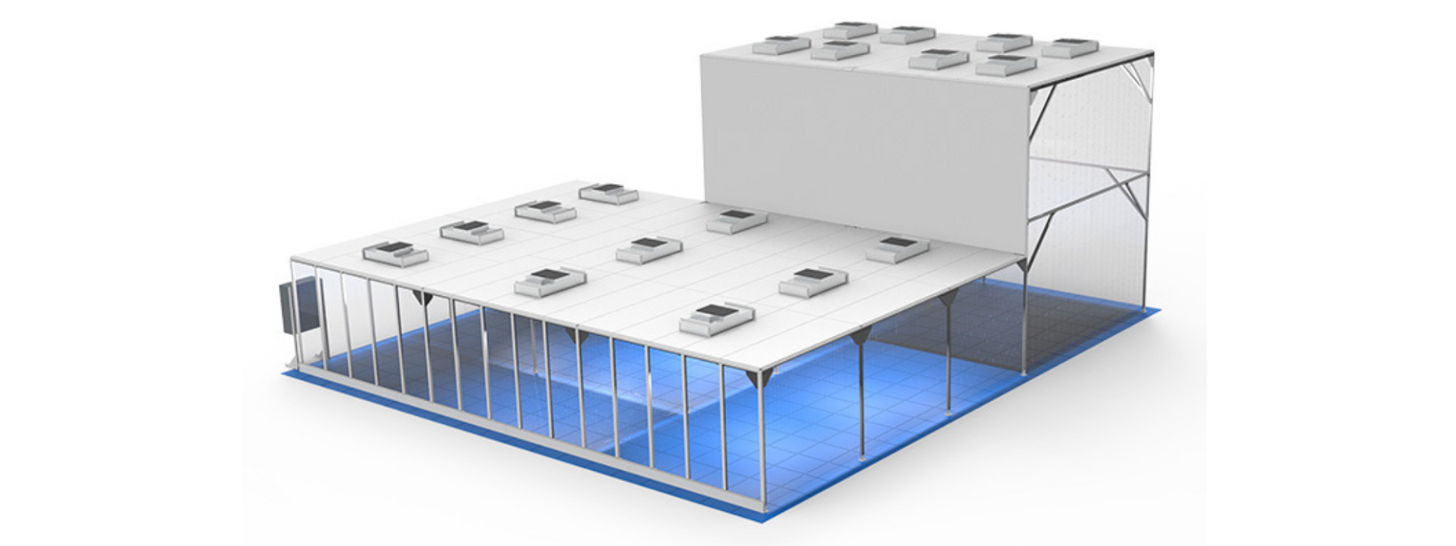
Battery dry rooms
- Single or multi-rotor low dewpoint AHU options to suit your budget, space, and specification needs.
- Bespoke, industry-leading solutions. Ideal for battery and semi-conductor manufacturing.

General & smart laboratories
- Specialist ancillary supplies, such as fixtures and fittings, benching & pass-through hatches.
- All specialist client equipment within the lab can be integrated for optimum compliance.

Biotech & containment suites
- We will assess the relevant biocontainment legislation and appropriate operational parameters for your use.
- Modular biocontainment suites available as independent structures.
What sets us apart?
With an end-to-end service, our multidisciplinary team design, deliver, and commission complete cleanroom systems.
- Full project management and ongoing maintenance
- Decades of experience in functional and mechanical system design
- Technical specification and reliability of our cleanrooms is second to none
With a UK network of offices, we are ideally placed to support customers across the country.

Our success stories
We apply creative thinking to modular cleanroom projects, resulting in functional spaces that achieve a significant return on investment. Learn how we add value to our clients through our case studies.

You'll be in good company
Frequently asked questions
We offer softwall, hardwall, and panel system as options for your cleanroom project. GMP cleanrooms will always need the flush panel system, but ISO cleanrooms are more flexible. The right construction type for you will depend on your process, but our Project Sales Engineers will be able to talk you through your options.
This depends on the construction type and class, but we can provide you with a rough order of magnitude (ROM) cost which gives you an estimated price range based on your requirements. Contact us to request your ROM price.
Every cleanroom design and build project will be validated during the commissioning phase. Then ISO 14644-2:2015 dictates that they need to be validated every 6-12 months depending on their class and your risk assessment. We have a dedicated after-sales and validations team who can talk you through your requirements.
Ultimately, this depends on your own process and risk assessment. If you aren’t sure, speak with your regulatory body and auditors to find out what their expectations are. We have a regulatory and governance team who will make sure your new cleanroom is designed to be compliant with your required ISO class or GMP grade.
Find out about the cell & gene therapy suite we built in Edinburgh
START A PROJECT WITH US
Our design and build specialists have experience working with customers in all kinds of industries on a global scale, achieving great results time and time again. We’d love to work with you as well!
CONTACT US